For adults with moderately to severely active ulcerative colitis who are steroid dependent or have failed or were intolerant to conventional therapy.
Take a fresh approach to UC with SIMPONI®
SIMPONI® is a prescription medicine and the first and only injectable UC treatment approved by the FDA to both control symptoms and begin to improve the appearance of the intestinal lining for moderately to severely active adult UC patients who have not responded well to certain other UC medications or find it necessary to continue taking steroid medications.
Ulcerative Colitis Treatment With SIMPONI®
While individual results may vary, SIMPONI® was proven in clinical studies to:
- Help people with moderately to severely active UC get their symptoms under control in as few as 6 weeks
- Begin to improve the appearance of the intestinal lining in as few as 6 weeks
- Keep symptoms under control with a single injection just once every 4 weeks for those people who responded to SIMPONI®
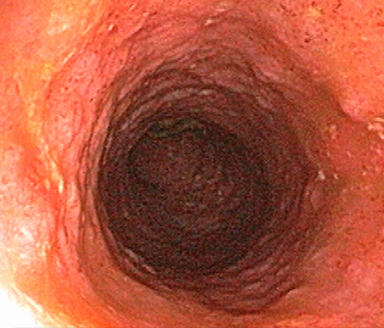
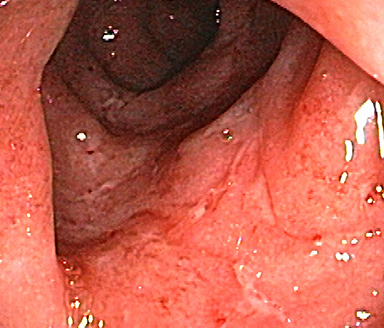
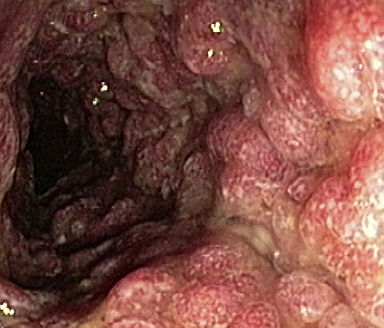
These photos are representative of colons diagnosed with varied severities of UC and not from patients taking SIMPONI®.
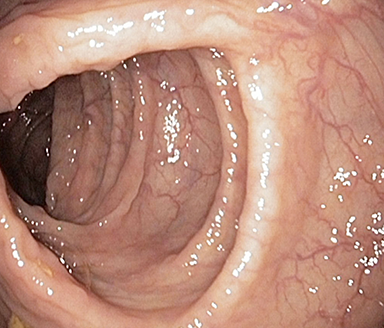
Healthy colon
Mild UC
Moderate UC
Severe UC
SIMPONI® is not for everyone. Only your doctor can decide if SIMPONI® is right for you.
Talk to your doctor to find out if SIMPONI® is right for you.

Talk to your doctor about UC symptom control and beginning to improve the appearance of the intestinal lining as possible treatment goals
As UC progresses, the appearance of the intestinal lining can become dry, brittle, and inflamed, and tiny sores can develop. Improving the appearance of the intestinal lining can be a realistic treatment goal in the management of UC. SIMPONI® is clinically proven to begin improving the appearance of the intestinal lining.
Meet Lindsey
Lindsey is a medical assistant from Georgia who works with UC patients being treated with SIMPONI®. She wants to share her experiences.
Interested in sharing your story?
Sign up

How does SIMPONI® work?

Before starting a new medication, it's important to know what it is, how it works, and what you can expect from treatment. Here's how SIMPONI® can help relieve the symptoms of moderately to severely active ulcerative colitis (UC).
The role of TNF-alpha

Tumor necrosis factor-alpha (TNF-alpha) is a protein made by your body's immune system. People with diseases such as UC have too much TNF-alpha. Excess amounts of TNF-alpha cause your immune system to mistakenly attack healthy cells in your gastrointestinal tract. This leads to inflammation, which causes the symptoms you experience with UC.
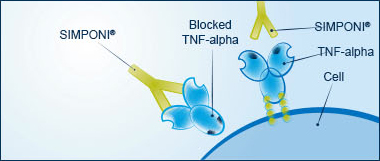
SIMPONI® targets, binds with, and blocks excess TNF-alpha, helping to control inflammation.
SIMPONI® targets TNF-alpha
SIMPONI® specifically targets excess TNF-alpha, helping to block an underlying cause of the symptoms of UC. SIMPONI® can bind with 2 forms of TNF-alpha, and at more than 1 location. It's important to know that blocking too much TNF-alpha can reduce your body's ability to fight infections.
Injectable UC treatment at a glance
Indication for Use
SIMPONI® is used for adults with moderately to severely active ulcerative colitis (UC) who are steroid dependent or have failed or were intolerant to conventional therapy.
SIMPONI®
(golimumab)
Humira®
(adalimumab)
Begin helping some of your symptoms

Get and keep your UC under control

Induce, achieve, and sustain remission

Induce and sustain remission
Begin to improve the appearance of your intestinal lining

SIMPONI®
(golimumab)

Humira®
(adalimumab)
SIMPONI®
(golimumab)

Induce, achieve, and sustain remission
Humira®
(adalimumab)

Induce and sustain remission
SIMPONI®
(golimumab)

Humira®
(adalimumab)
This chart is not intended to compare the safety, effectiveness, or uses of these treatments. Please talk with your doctor regarding each treatment.
SIMPONI® is a prescription medicine used to begin helping some of your symptoms, to begin improving the way the lining of your large intestine looks to your doctor during colonoscopy, and in people who respond to SIMPONI® to get their ulcerative colitis (UC) under control (induce remission) and keep it under control (sustain remission). SIMPONI® is used in adults with moderately to severely active UC when certain other UC medicines have not worked well enough or cannot be tolerated, or if it is necessary to continue taking steroid medicines.
Humira® is a prescription medicine used in adults to help get moderate to severe UC under control (induce remission) and keep it under control (sustain remission) when certain other medicines have not worked well enough. It is not known if Humira® is effective in people who stopped responding to or could not tolerate anti-TNF medicines. Humira® is a registered trademark of AbbVie Inc.
Treatment Frequency
Number of injections in the first year of treatment: SIMPONI® vs Humira®
Getting Started
SIMPONI®
(golimumab)
![]()
Day 1
Day 15
Humira®
(adalimumab)
![]()
Day 1
Day 15
Maintaining Treatment
1 injection every 4 weeks
SIMPONI®
(golimumab)

1 injection every 4 weeks
1 injection every 2 weeks
Humira®
(adalimumab)

1 injection every 2 weeks
This chart is not intended to compare the safety, effectiveness, or uses of these treatments. Please talk with your doctor regarding dosing.
Treatment with SIMPONI® begins with 3 starter injections: 2 injections on your first day of treatment, followed by 1 injection 2 weeks later. After these 3 starter injections, SIMPONI® requires just 1 injection every 4 weeks. If your doctor decides that you or your caregiver can give your injections at home, you will need to be trained on the proper way to self-inject SIMPONI® directly under the skin. Once you've learned how to self-inject, you can do it at home without visiting a doctor's office.
SIMPONI® is intended for use under the guidance and supervision of a physician. Patients may self-inject with SIMPONI® after physician approval and proper training.
Prior to initiating SIMPONI® and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection. Prior to initiating SIMPONI®, patients should be tested for hepatitis B viral infection.
While dosing frequency is important, there are other factors to consider.
Talk to your doctor about which treatment is right for you.
SIMPONI® (golimumab) Other Important Considerations
SIMPONI® is a medicine that affects your immune system. SIMPONI® can lower the ability of your immune system to fight infections. Some people have serious infections while taking SIMPONI®, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that spread throughout their body. Some people have died from these serious infections.
- Your doctor should test you for TB and hepatitis B before starting SIMPONI®.
- Your doctor should monitor you closely for signs and symptoms of TB during treatment with SIMPONI®.
You should not start taking SIMPONI® if you have any kind of infection unless your doctor says it is okay.
To learn more about these and other risks, please read the Important Safety Information about SIMPONI® below, and the Medication Guide, and talk with your doctor.
